
:max_bytes(150000):strip_icc()/BoronGroup-56a12d305f9b58b7d0bcccb4.png)
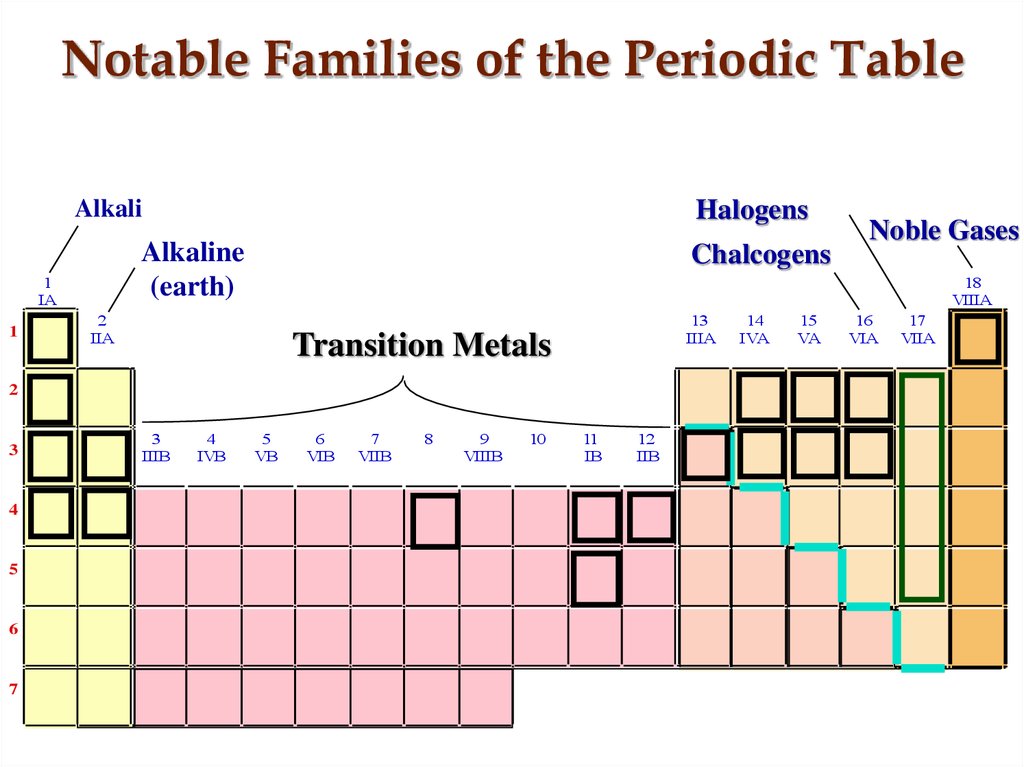
Since the second period of p block elements like boron, carbon, nitrogen, oxygen, fluorine, and argon have filled s-orbitals with valence shell electron configuration, 2s 2 2p 1→6, where n = number of period. Group-3, 4, 5, 6, 7, and noble gases belong to p-block elements. P-block on the periodic table organizes by progressively filled p-orbital in valence shell electronic structure but helium is an exception with electron arrangement 1s 2. The valence shell electron configuration, ns 1→2, where n = principle quantum number, or the number of periods. Group-1 (hydrogen, lithium, sodium, potassium, rubidium, cesium, and francium) and group-2 (beryllium, magnesium, calcium, strontium, barium, and radium) belong to s-block elements.
/769723031-1024x1024-5b86a9c746e0fb0025177f44.jpg)
The name s-block element in the periodic table is given due to the arrangement of electrons, the valence electron enters into ns-orbital and is filled progressively according to the configuration rules. The organization of the middle portion of the periodic table made the relationship between the left and right sections and contains a list of transition metals or d-block and inner transition or f-block elements.Īccording to the modern periodic law based on the atomic number and valence shell electron arrangement of elements, the different types of metals and non-metals in chemistry are organized to form s, p, d, and f-block on the periodic table.


 0 kommentar(er)
0 kommentar(er)
